Separation by Distillation
Crude oil has been described as a complex mixture of hydrocarbons. It has no use in its raw form. Therefore, it needs to be separated into its different products called fractions. This is done by a process called fractional distillation. Before covering this, the simple process of distillation will be explained first.
Distillation is a separation technique in which a pure liquid is separated from a mixture of liquids. The distillation process consists of the following main steps:
- Heating
- Evaporating
- Cooling
- Condensing
A mixture of ethanol (an alcohol) and water will be used to explain the process of distillation. The separation technique makes use of the difference in boiling points between the liquids in the mixture. However, before moving onto distillation it is important to gain an understanding on the key intermolecular forces at play. Both ethanol and water have strong intermolecular forces called hydrogen bonds. Without knowledge of hydrogen bonding one might deduce that ethanol with its greater molecular weight and size should have stronger intermolecular forces and therefore a higher boiling point than water. However, this is not the case due to hydrogen bonding.
Hydrogen Bonding
Intermolecular forces in compounds arise due to an imbalance of charge. For Van der Waal forces this is a temporary imbalance caused due to the constant movement of electrons in an atom at high speeds.
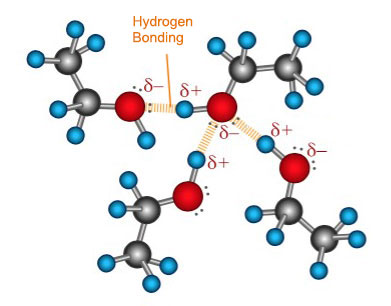
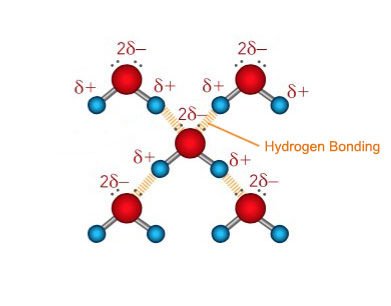
Hydrogen bonding is caused by the ability of particular atoms to strongly attract the electrons in a bond. A measure of how strongly an atom in a compound attracts electrons in a bond is called electronegativity. Hydrogen bonds are formed when a hydrogen atom is covalently bonded to one of the electronegative atoms, fluorine, chlorine, oxygen or nitrogen.
In water the hydrogen bond is formed due to the covalent bonds between one oxygen atom and two hydrogen atoms. Oxygen is a highly electronegative atom and attracts the electrons in the O—H bonds towards itself. Since the proton in the nucleus of the hydrogen atom is only slightly screened the action of the oxygen pulling the electrons away from the hydrogen results in a net positive charge on the hydrogen atoms. Consequently there is also a net negative charge on the oxygen atom resulting in an imbalance of charge across the water molecule. The overall water molecule is said to be polar because like a magnet it has two opposite charges on either ends (its poles). The net positive hydrogen atoms are readily available to attract the negative electron clouds from the oxygen atoms in adjacent water molecules. Unlike Van der Waal forces, hydrogen bonding involves a permanent imbalance of charge and therefore results in permanent dipole attractions. Hydrogen bonds are stronger than Van der Waal forces but weaker than covalent bonds. In water there are two O—H bonds and therefore each water molecule can form two hydrogen bonds. Each ethanol molecule on the other hand has only one O—H bond and thus forms one hydrogen bond with adjacent ethanol molecules.
The animation below shows hydrogen bonding in water molecules.
It is the increased number of hydrogen bonding in water that gives it a higher boiling point than ethanol. It is also due the hydrogen bonding between the ethanol and water molecules that the two liquids are miscible at all proportions.
| Hydrogen Bonding in Water | Hydrogen Bonding in Ethanol |
|---|---|
 |
 |
 |
|
