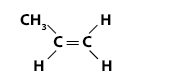Addition Polymerisation
Addition polymerisation involves addition reactions in which a large number of small molecules (monomers) join together to form very large molecules (polymers).
In addition polymerisation the monomers have at least one double bond between carbon atoms. Alkenes are particularly useful monomers as they contain double bonds and can be made to undergo addition reactions amongst themselves.
Addition reactions involving alkenes are reactions in which the carbon-carbon double bond is converted to a single bond and atoms or group of atoms are added to each of the two carbon atoms.
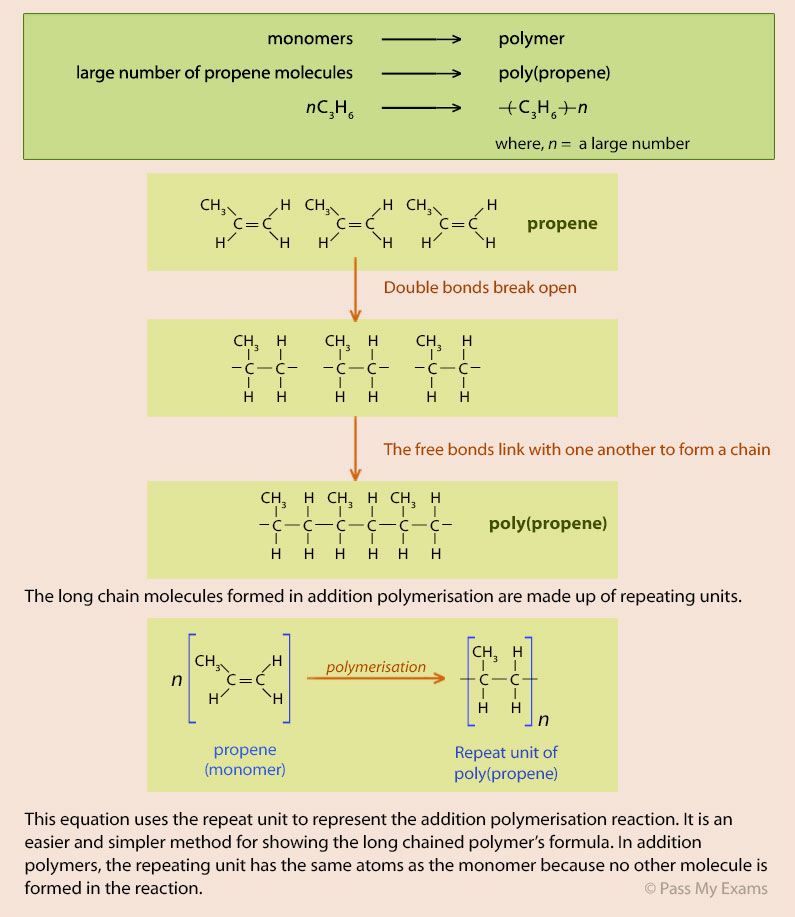
Example 1 – Poly(ethene)
Poly(ethene) or polythene is one of the most commonly used synthetic polymers. It is produced by the addition polymerisation of small ethene molecules which are the monomers in the reaction.
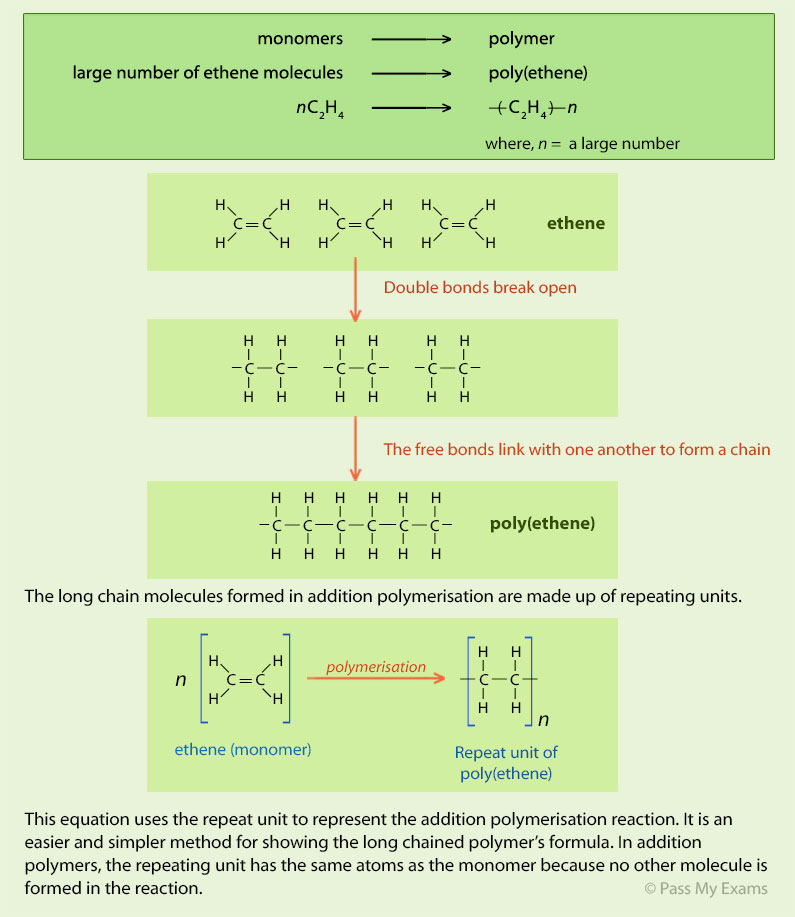
Example 2 – PVC
Poly(chloroethene) also known as poly vinyl chloride (PVC)
Chloroethene (vinyl chloride) undergoes addition polymerisation to form poly(chloroethene) which is more commonly known by the initials of its old name, poly-vinyl chloride (PVC).
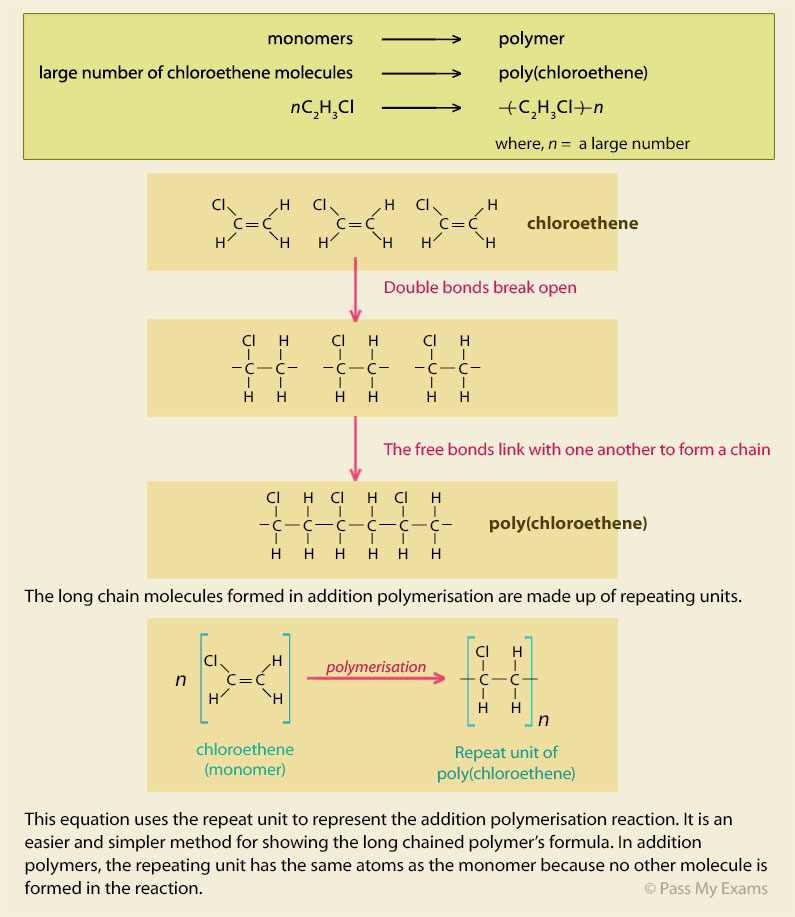
Poly(chloroethene) or PVC is a fairly strong material due to considerable intermolecular forces produced by the polar C – Cl bond. It is also a versatile material which is water and fire resistant. These properties mean it has a lot uses and is a major product produced by the chemical industry. On its own, poly(chloroethene) is a fairly rigid plastic, but additives called plasticisers are added to it to make more softer and flexible. Poly(chloroethene) or PVC has numerous uses from water pipes, guttering, window and door profiles, fabric coatings, electrical wire and cable insulation, furniture etc.
Example 3 – Poly(tetrafluoroethene) PTFE
Tetrafluoroethene undergoes addition polymerisation to form poly(tetrafluoroethene) which is also known under the brand names of Teflon or Fluon.
Tetrafluoroethene is ethene molecule in which all the hydrogen atoms have been replaced by fluorine atoms (4 fluorine atoms to an ethene molecule, giving the name tetrafluoroethene). Structurally, PTFE is the same as poly(ethene) except that the hydrogen atoms are replaced by fluorine atoms.
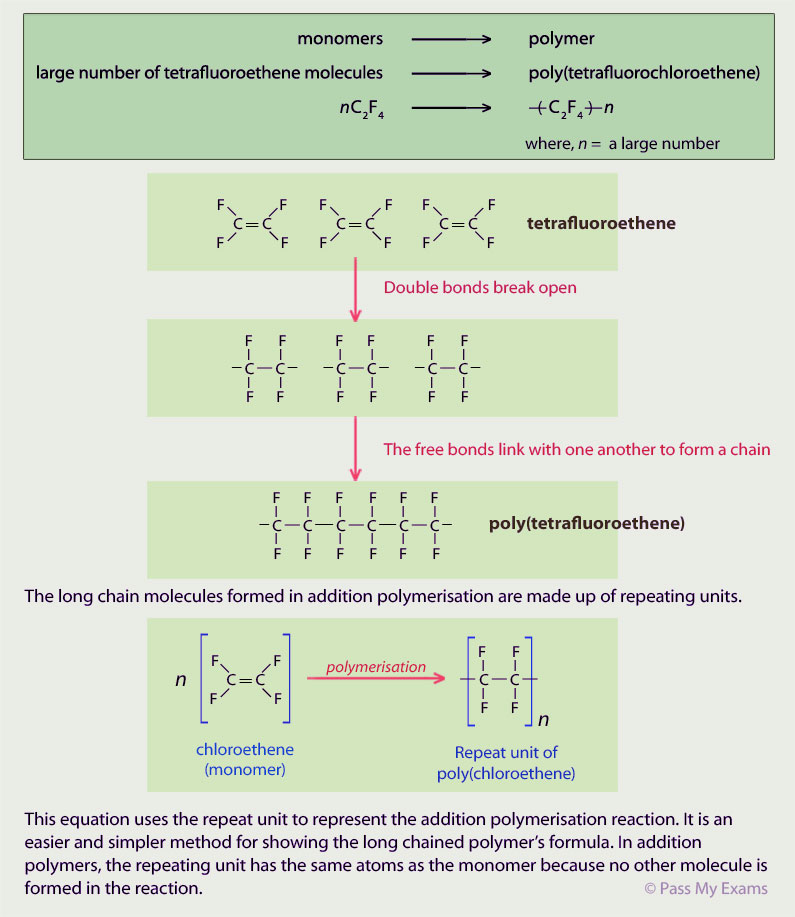
Poly(tetrafluoroethene) is very resistant to chemical attack. The fluorine atoms act as a shield for the carbon chain in the polymer preventing anything from reacting with it. This chemical resistant property makes it useful in the chemical and food industry as a coating material. Vessels and pipes can be coated with PTFE to make them resistant to chemicals that might attack and corrode them. PTFE also has non-stick properties and this property has been used to great success in non-stick kitchen utensils.
Example 4 – Poly(propene) (polypropylene)
Propene is an alkene readily available from petroleum. It can undergo addition polymerisation to produce poly(propene).
Propene has the molecular formula C3H6 and the structural formula:
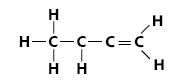
In order to represent the polymerisation the propene monomer is redrawn as below to make it easier to represent it as a repeat unit: