Penetrating Properties of Radiation
Radiations from radioactive materials can be dangerous and pose health hazards. By knowing the ability of the different types of radiation to penetrate matter allows us to gain an understanding on how best to protect ourselves.
The animation below shows the penetrating properties of radiations.
Penetration of Alpha Particles
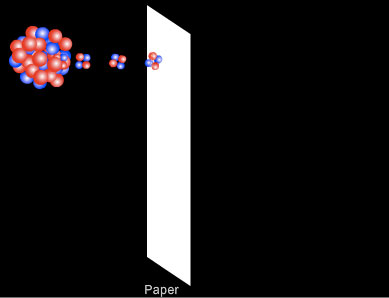
Alpha particles can be absorbed by a thin sheet of paper or by a few centimetres of air. As alpha particles travel through air they collide with nitrogen and oxygen molecules. With each collision they lose some of their energy in ionising the air molecule until eventually they give up all of their energy and are absorbed. In a sheet of paper the molecules are much close together so the penetration of alpha particles is much less than in air.
Penetration of Beta Particles
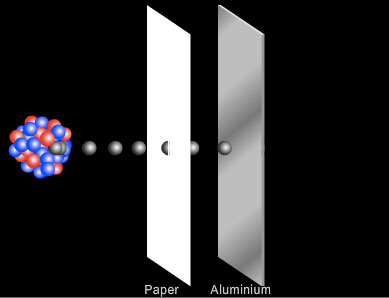
Beta particles travel faster than alpha particles and carry less charge (one electron compared to the 2 protons of an alpha particle) and so interact less readily with the atoms and molecules of the material through which they pass. Beta particles can be stopped by a few millimetres of aluminium.
Penetration of Gamma rays
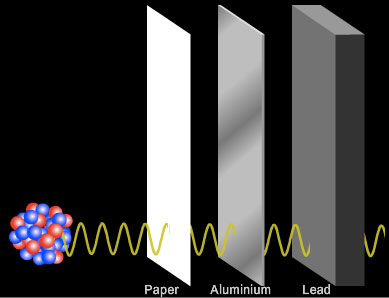
Gamma rays are the most penetrating of the radiations. Gamma rays are highly energetic waves and are poor at ionising other atoms or molecules. It cannot be said that a particular thickness of a material can absorb all gamma radiation. Many centimetres of lead or many meters of concrete are required to absorb high levels of gamma rays.
