Flame colours of alkali metals
Alkali metals emit distinctive flame colours when heated. These flame colours are used to identify these elements.
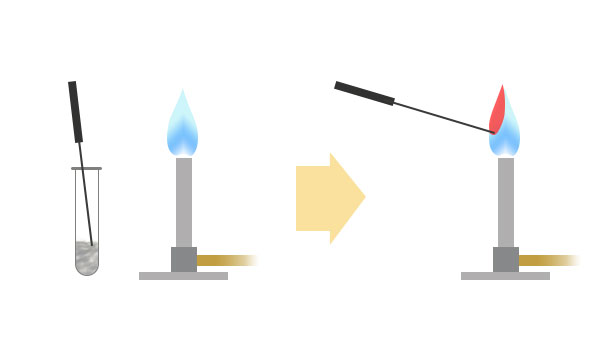
A small piece of metal compound is taken on the end of a Nichrome wire and introduced into a Bunsen flame. The flame emitting from the end of wire will show a distinctive colour that is characteristic of the metal in the compound. By referring that flame colour to the table below, we can identify the element in that compound.
Flame colours of Group 1 elements
| Element | Flame colour |
|---|---|
| lithium | red |
| sodium | yellow |
| potassium | lilac |
Line spectra
All atoms give off light when heated, although this light is not always visible to the human eye. A technique, known as spectroscopy, is used to split this light to form a line spectrum. Each element has its own distinctive line spectrum which is used to identify these elements. For example, the element helium was discovered by studying line spectra emitted by the Sun.
A line spectrum is shown below:
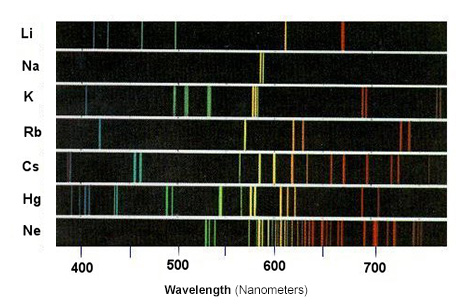
The study of line spectra helped scientists discover some elements which was not possible until the development of spectroscopy. Some elements, such as rubidium and caesium, were discovered through spectroscopy.
