Ionic Compounds
An ionic compound is a giant structure of ions. Strong electrostatic forces of attraction between oppositely charged ions hold ionic compounds together. These forces, called ionic bonding, act in all directions in the lattice.
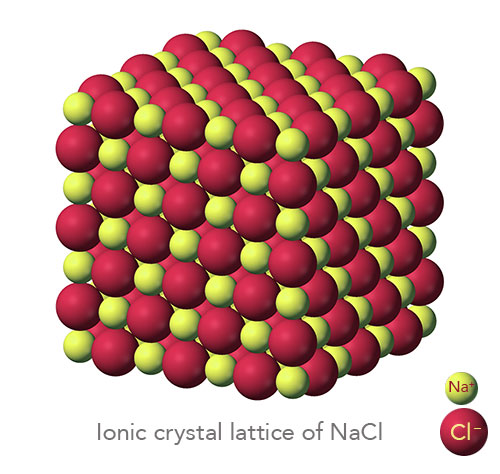
The number of ions in an ionic compound is such that the overall charge of a sample of the compound is zero.
Properties of Ionic Compounds
High melting and boiling points
The ions in a crystal lattice are very strongly bonded together, so a high temperature is required to separate the ions and melt the crystal. These compounds have high melting points and high boiling points because of the large amounts of energy needed to break the many strong bonds.
Conductivity
Ionic compounds do not conduct electricity when solid. The ions are held strongly in position, so they cannot move and carry an electric current. When melted or dissolved in water, ionic compounds conduct electricity because the ions are free to move and carry the current. e.g. Sodium chloride is soluble in water and the solution conducts electricity.
