Radiocarbon dating
Radiocarbon or Carbon-14 dating is a technique used by scientist to date bones, wood, paper and cloth.
Carbon-14 is a radioisotope of Carbon. It is produced in the Earth’s upper atmosphere when Nitrogen-14 is broken down to form the unstable Carbon-14 by the action of cosmic rays. The unstable Carbon-14 is transported down to the lower atmosphere by atmospheric activity such as storms.
Carbon-14 reacts identically to Carbon-12 and is rapidly oxidised to form (Carbon-14)Dioxide. Since all living organisms on Earth are made up of organic molecules that contain Carbon atoms derived from the atmosphere, they therefore contain Carbon-14 atoms. The Carbon-14 within a living organism is continually decaying, but as the organism is continuously absorbing Carbon-14 throughout its life the ratio of Carbon-14 to Carbon-12 atoms in the organism is the same as the ratio in the atmosphere. Once an organism dies it stops taking in Carbon in any form. The unstable Carbon-14 within the organism begins to decay to form Nitrogen-14 by emitting a beta particle. Over time there is a gradual decrease in the amount of Carbon-14 and the ratio of Carbon-14 atoms to other Carbon atoms declines.
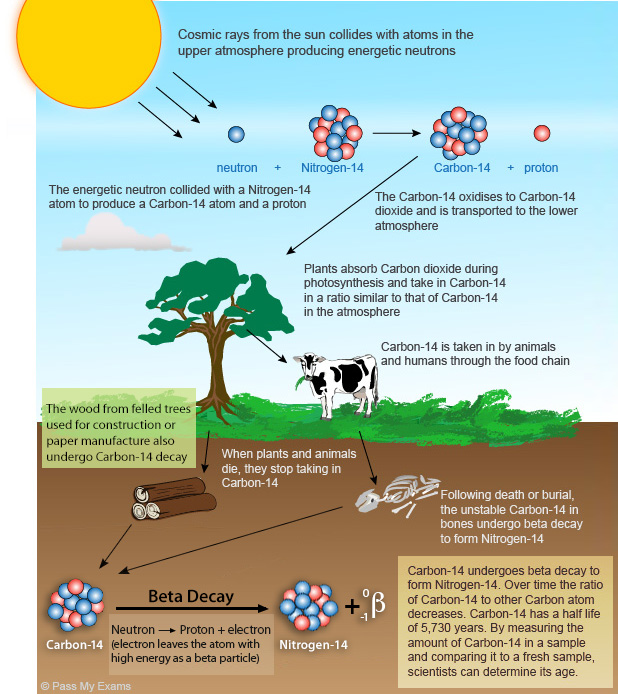
The half life for Carbon-14 is 5730 years. Therefore half of the Carbon-14 has decayed after 5730 years. Half of the remaining Carbon-14 then decays over the next 5730 years leaving one fourth of the original amount. By measuring the ratio of Carbon-14 in a sample and comparing it to the amount in a recently deceased sample its date can be determined.
