Addition Reaction of Alkenes with Halogens
Alkenes contain at least one carbon-carbon double bond. It is the presence of this double bond that makes alkenes more reactive than alkanes. As was demonstrated by the test for alkenes using bromine water, all alkenes can be characterised by their addition reactions. Addition reactions involving alkenes are reactions in which the carbon–carbon double bond is converted to a single bond and atoms or groups are added to each of the two carbon atoms.
Alkenes undergo an addition reaction with halogens; the halogen atoms partially break the carbon-carbon double bond in the alkene to a single bond and add across it.
Remember the reactivity of halogens decreases down the group, therefore the addition reaction of alkenes with chlorine takes place faster with chlorine than for bromine with iodine being the slowest. Fluorine is the most reactive of all non-metals elements and does not produce a useful reaction with alkenes. For example ethene reacts explosively with fluorine to produce carbon and hydrogen fluoride gas. Below are the chemical equations for the first four members of the alkenes homologous series addition reactions with halogens.
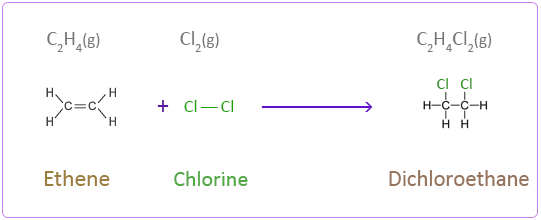
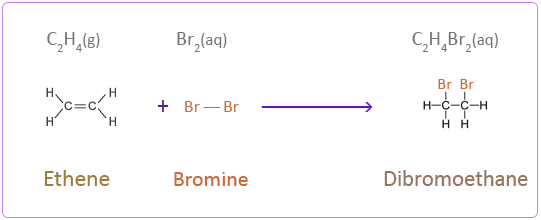
Addition Reactions of Ethene with Halogens
Chlorine
Bromine
Iodine
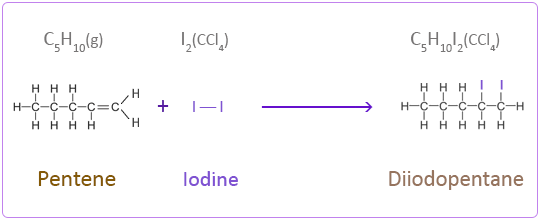
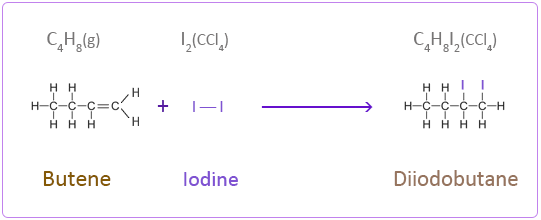
Iodine is dissolved in a solvent such as tetrachloromethane (CCl4).

Addition Reactions of Propene with Halogens
Chlorine
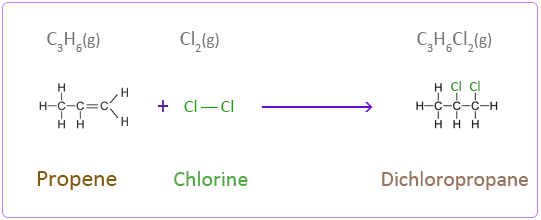
Bromine
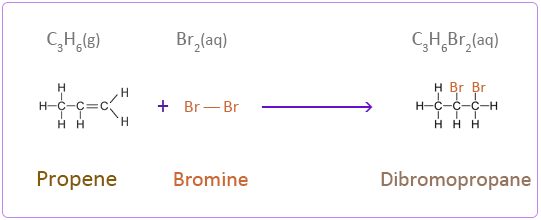
Iodine
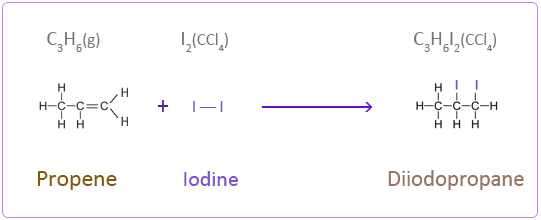
Addition Reactions of Butene with Halogens
Chlorine
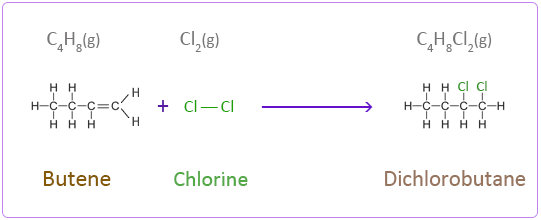
Bromine
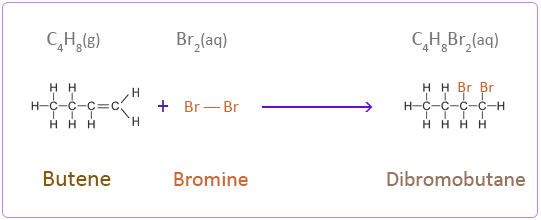
Iodine
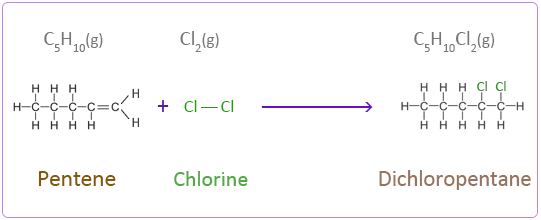
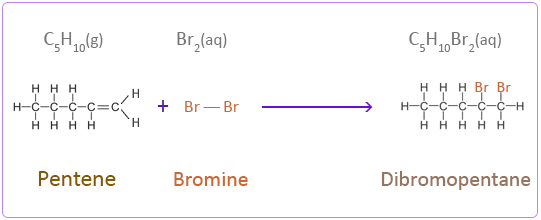
Addition Reactions of Pentene with Halogens
Chlorine
Bromine
Iodine