Addition Reaction of Alkenes with Water
Alkenes contain at least one carbon-carbon double bond. It is the presence of this double bond that makes alkenes more reactive than alkanes. As was demonstrated by the test for alkenes using bromine water, all alkenes can be characterised by their addition reactions. Addition reactions involving alkenes are reactions in which the carbon–carbon double bond is converted to a single bond and atoms or groups are added to each of the two carbon atoms.
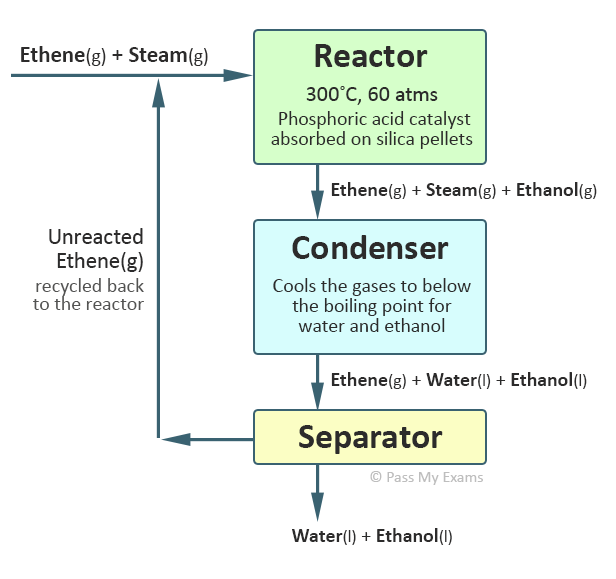
Alkenes undergo an addition reaction with water in the presence of a catalyst to form an alcohol. This type of addition reaction is called hydration. The water is added directly to the carbon – carbon double bond. The hydration of alkenes can be undertaken by various methods. Traditionally in industry a strong acid such as sulphuric acid is used to produce an intermediate product which is then added to water and warmed to produce an alcohol. The acid is regenerated after the reaction and therefore acts like a catalyst. More recently direct catalytic hydration is used particularly for converting ethene to ethanol. In this process ethene and steam (water in the gaseous phase) are passed at 300°C and a pressure approximately 60 times above atmospheric pressure over a phosphoric acid catalyst to produce ethanol. This reaction is reversible with only 5% of the ethene converting to ethanol with each pass over the catalyst in the reactor. However, by collecting the gases from the reactor and cooling them the ethanol and water will their higher boiling points will condense and can be separated as liquids from the unreacted ethene gas which is recycled back to the reactor. This allows an overall conversion of ethene to ethanol of 95%.
Catalytic Hydration of Ethene
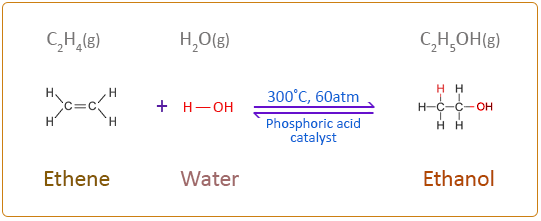
A simple flow diagram of the process diagram is given below:
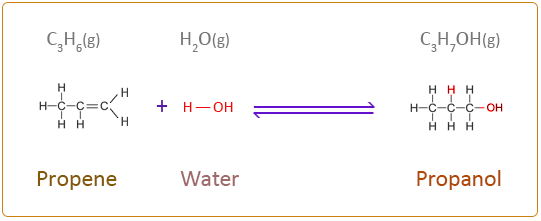
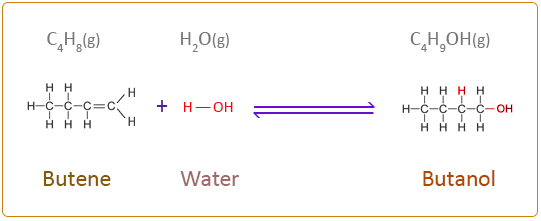
The chemical equation for the next three members of the alkene homologous series addition reaction with water is given below. When writing the structural formula for this type of reaction it is best to consider the water molecule as H–OH.
Propene
Butene
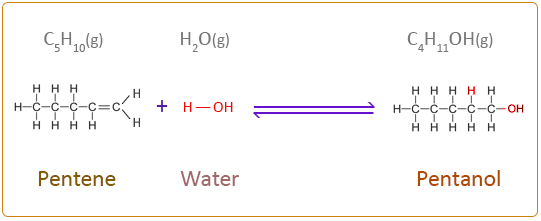
Pentene