Chemical Compounds
When atoms of two or more elements are chemically combined, they form substances called compounds. These compounds have different types of properties depending on the type of chemical bonding involved.
Covalent Compounds
Covalent bonds create substances consisting of Simple Molecules such as Hydrogen (H2), Oxygen (O2), water (H2O), or giant structures (Macromolecules), such as diamond and silicon dioxide SiO2.
Simple covalent compounds (Simple Molecules)
Molecules are groups of two or more atoms joined together by chemical bonds. Simple molecules are formed when two or more atoms join together by covalent bond.
A water molecule is formed when two hydrogen atoms bond with an oxygen atom.
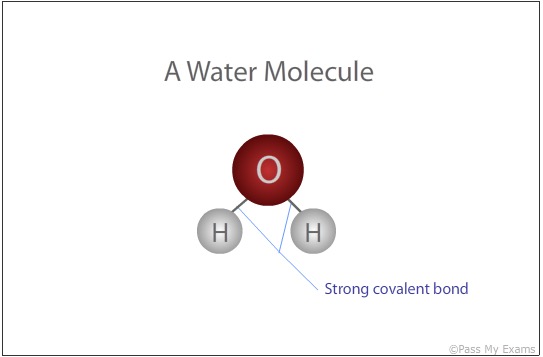
Examples of Simple covalent compounds
Hydrogen (H2), Carbon Dioxide (CO2), Chlorine (Cl2), Hydrogen Chloride (HCl), ammonia (NH3), and Methane (CH4) are some examples of simple molecules.
Click to view how simple molecules are formed by covalent bonding.
Properties of simple covalent compounds:
Low melting and boiling points
Substances that consist of simple molecules are gases, liquids or solids with low melting and boiling points.
View the animation below to see why they have low melting and boiling points.
Conductivity of simple covalent compounds
Substances that consist of simple molecules are non-conductor of electricity, because the molecules do not have an overall electric charge or free electron.
