Alkenes & Alkenes Homologous Series
Alkenes are referred to as unsaturated molecules. This means they contain at least one carbon-carbon double bond which displaces two hydrogen atoms and thus, alkenes do not have the maximum number of hydrogen atoms per carbon atom.
Alkene Homologous Series
The alkenes form a homologous series. A hydrocarbon homologous series is a series of hydrocarbons which:
- Have the same general formula
- Differ by CH2 in molecular formulae from neighbouring compounds
- Show a gradual variation in physical properties i.e. boiling and melting point
- Have similar chemical properties
The general formula for the homologous series of alkenes is CnH2n where n is the number of carbon atoms. Because alkenes are hydrocarbons with at least one carbon-carbon double bond the alkene homologous series starts at ethene C2H4.
The compounds are named as for the alkanes, but with the ending, “ene” instead of “ane”.
Each successive molecule in the alkene homologous series is formed by adding a carbon and two hydrogen atoms or a CH2 (methylene group) to the previous molecule. The incremental change in relative molecular mass is therefore fourteen.
The table below shows the first four straight chained alkenes in the alkene homologous series. In the structural formula the carbon-carbon double bond is represented by two lines between the two carbon atoms.
| Name | Number of Carbon atoms | Molecular Formula CnH2n |
Structural Formula |
|---|---|---|---|
| ethene | 2 | C2H2(2) = C2H4 | 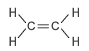 |
| Propene | 3 | C3H2(3) = C3H6 | 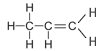 |
| Butene | 4 | C4H2(4) = C4H8 | 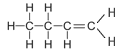 |
| Pentene | 5 | C5H2(5) = C5H10 | 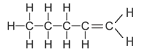 |
