Test for Alkenes
Alkenes are called unsaturated compounds. They contain at least one carbon-carbon double bond which displaces two hydrogen atoms and so do not have the maximum number of hydrogen atoms per carbon atom.
Alkanes on the other hand are called saturated compounds. They contain only single bonds and the maximum number of hydrogen atoms per carbon atom.
The presence of the double bond in alkenes makes them more reactive than alkanes. This higher reactivity of the alkenes over alkanes allows us to distinguish them. A simple test with bromine water can be used to tell the difference between an alkane and an alkene. An alkene will turn brown bromine water colourless as the bromine reacts with the carbon-carbon double bond. In fact this reaction will occur for unsaturated compounds containing carbon-carbon double bonds. An alkane undergoes no reaction with bromine water and therefore there is no colour change.
The diagram below highlights the test to distinguish between alkanes and alkenes or saturated or unsaturated hydrocarbon compounds.
 |
 |
|
 |
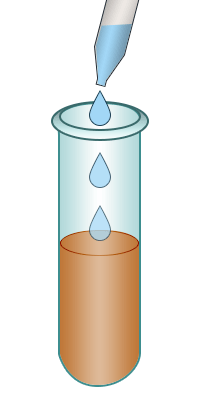
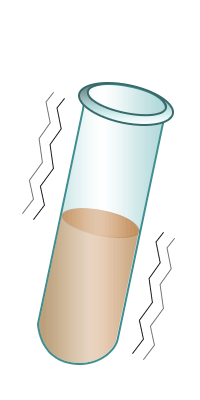
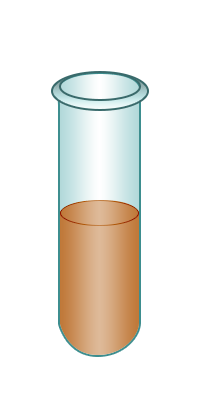
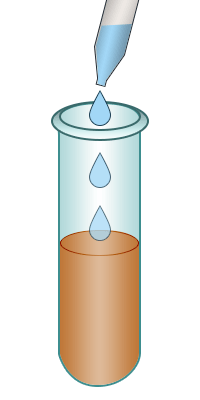
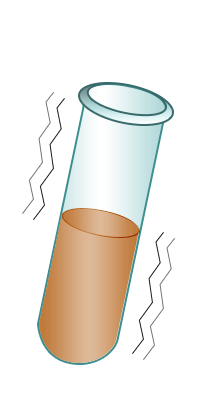
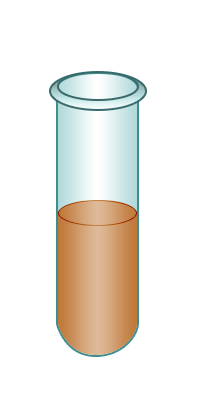
| Test-tube containing bromine water. | Add the alkene to the bromine water. | Shake. | The bromine water turns colourless confirming the presence of an alkene. |
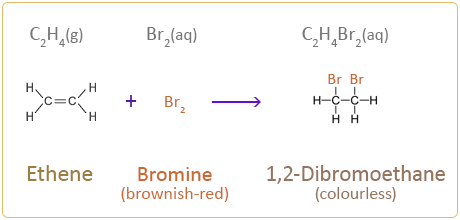
Although ethene is a gas at room temperature, it is used below as an example to show the reaction between an alkene and bromine taking place: C2H4(g) + Br2(aq) → C2H4Br2(aq) The bromine atoms break the carbon–carbon double bond and from C–Br bonds. This type of reaction is called an addition reaction and the addition of bromine to an alkene is called bromination. |
|||
|
|||
 |
 |
 |
 |
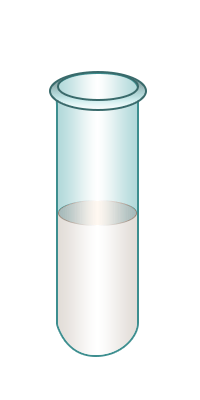
| Test-tube containing bromine water. | Add the alkane for to the bromine water. | Shake. | No change in colour to bromine water is observed confirming the presence of an alkane. |
Alkanes are saturated and contain single bonds. They do not undergo any reaction with bromine.
|
|||