Carboxylic Acids Solubility in Water
The first four carboxylic acids mix with water in all proportions. This high solubility of water can be explained by hydrogen bonding (the same reason as for alcohols).
Hydrogen bonding is caused by the ability of particular atoms to strongly attract the electrons in a bond. A measure of how strongly an atom in a compound attracts electrons in a bond is called electronegativity. Hydrogen bonds are formed when a hydrogen atom is covalently bonded to one of the electronegative atoms, fluorine, chlorine, oxygen or nitrogen.
Carboxylic acids contain the carboxyl group (COOH or CO2H). The carboxyl group is made up of a carbonyl group (C=O) and a hydroxyl group (O—H). Like alcohols, in carboxylic acids the hydrogen bonds are formed due to the covalent bonds between one oxygen atom and one hydrogen atom in the hydroxyl group (O—H). Oxygen is a highly electronegative atom and attracts the electrons in the O—H bonds towards itself. Since the proton in the nucleus of the hydrogen atom is only slightly screened the action of the oxygen pulling the electrons away from the hydrogen results in a net positive charge on the hydrogen atom. Consequently there is also a net negative charge on the oxygen atom resulting in an imbalance of charge across the hydroxyl group. The overall hydroxyl group is said to be polar because like a magnet it has two opposite charges on either ends (its poles). The net positive hydrogen atom is readily available to attract the negative electron clouds from the oxygen atom in an adjacent carbonyl group. Unlike Van der Waals’ forces, hydrogen bonding involves a permanent imbalance of charge and therefore results in permanent dipole attractions.
The diagram below represents hydrogen bonding in ethanoic acid molecules. Each ethanoic acid molecule has one O—H bond but is capable of forming double hydrogen bonded dimers as shown in the diagram below.
| Hydrogen Bonding between Ethanoic Acid Molecules (CH3COOH) |
|---|
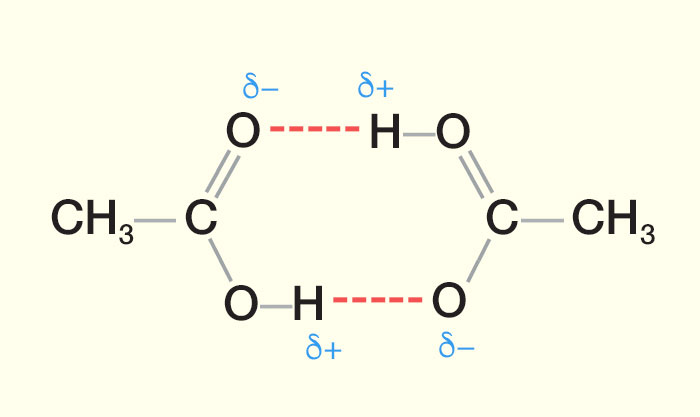 |
| In a pure carboxylic acid two molecules are bonded together using two hydrogen bonds to produce a dimer. A dimer consists of two identical molecules bonded together. The hydrogen bonds are formed between the slightly positive hydrogen atom in the hydroxyl group and the slightly negative oxygen in the carbonyl group. The dimer formed due to the hydrogen bonds doubles the size of the molecule thereby increasing the Van Der Waals’ intermolecular forces. This results in carboxylic acids having higher boiling points when compared to other organic compounds of similar carbon numbers or molecular masses. |
In water
When added to water the carboxylic acids do not form dimers. Rather, hydrogen bonds are formed between the individual molecules of the acid and water molecules. It is because of these interactions that carboxylic acids can dissolve in water to form acidic solutions.
The carboxylic acids with low molar mass up to four carbon atoms are freely soluble in water. As the chain length of the carboxylic acids increases the solubility in water decreases rapidly.
The animation below shows the interaction between ethanoic acid and water molecules in order to explain the solubility of small chain alcohols.
