Physical properties of Halogens
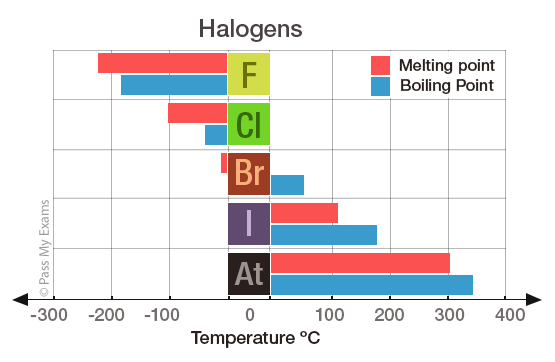
The table below summarises the physical properties of the first four Group 7 elements:
| Element | Electron config. |
State at room temp. (20°C) | Colour | Melting Point °C |
Boiling Point °C |
|---|---|---|---|---|---|
| Fluorine | 2.7 | Gas | Pale yellow | -220 | -188 |
| Chlorine | 2.8.7 | Gas | Green | -101 | -35 |
| Bromine | 2.8.18.7 | Liquid | Orange/Red | -7 | 59 |
| Iodine | 2.8.18.18.7 | Solid | Grey/Black | 113 | 183 |
Predictions in properties
Colour
From the table of physical properties it can be inferred that the depth of colour of the halogens increases in atomic number. Fluorine is pale yellow, chlorine is green, bromine is orange and iodine is grey. Thus the colour of the Astatine not included in the table can be deduced as being black.
Melting point and boiling point
The halogens belong to non-metals, and thus like typical non-metals they have low melting points and boiling points. The melting points and boiling points increase as you go down the group. Fluorine has the lowest melting point and boiling point.
The increase in melting point and boiling point can be explained by understanding Van Der Waal forces. Although this topic is not required at GCSE level it is thought a brief introduction would be useful in order to explain the trends in melting points and boiling points in the Group 7 elements.
Van der Waals’ forces are forces that exist between molecules and are therefore referred to as intermolecular forces. They are much weaker and different to the forces of attraction that exist between the bonds (ionic or covalent) of the atoms in a molecule which are referred to as intramolecular forces.
Van der Waals’ Forces
Van der Waals’ forces are named after the Dutch scientist J D Van der Waal who discovered them. The forces are created by the constant movement of electrons in atoms of molecules at high speeds. At any instant in time there is the likelihood that one side of the molecule has a greater proportion of electrons. When this happens a temporary imbalance of the electrons result in the molecule producing a negative and positive end. An atom or molecule that has an imbalance in charge is called a dipole and a temporary imbalance in charge produces a temporary dipole. The temporary imbalance in charge or temporary dipole can attract the electron cloud from a neighbouring atom or molecule i.e. the slightly positive end of the temporary dipole will attract the electrons from a neighboring molecule. This means the temporary dipole induces an imbalance of the electrons in the neighbouring molecule.
The strength of the Van de Waals’ force is dependent on the size of the atoms or molecules. As the size and molecular mass of the molecule increases so does the number of electrons resulting in a greater imbalance in charge and hence a stronger Van der Waals’ attraction. This is the reason why the melting point and boiling points increase down Group 7, more energy is required to overcome the stronger Van der Waals’ forces of attraction.
