Alkanes & the Alkane Homologous Series
Crude oil is a complex mixture of hydrocarbons. Hydrocarbons are chain molecules of varying lengths made from hydrogen and carbon atoms only, joined together by covalent bonds. Covalent bonds are formed when atoms share pair of electrons.
Most of the hydrocarbons in crude oil are hydrocarbons called alkanes. Alkanes are referred to as saturated molecules. The term saturated means they contain the maximum number of hydrogen possible, with no double or triple bonds between the carbon atoms. Therefore, alkanes only contain single bonds.
Alkane Homologous Series
The alkanes form a homologous series. A hydrocarbon homologous series is a series of hydrocarbons which:
- Have the same general formula
- Differ by CH2 in molecular formulae from neighbouring compounds
- Show a gradual variation in physical properties i.e. boiling and melting point
- Have similar chemical properties
The general formula for the homologous series of alkanes is CnH2n+2 where n is the number of carbon atoms.
All alkanes end in, “ane”. The first four alkanes in the homologous series retain their original names. After these the names are formed by adding the ending -ane to the Greek word for the number of carbon atoms in the molecule. For example pentane is formed from the word pent (Greek for five) and the suffix -ane, and hexane from hex (Greek for six) and the ending -ane. Thus, the first part of the name indicates the number of carbon atoms and the ending that it is an alkane.
Each successive molecule in the alkane homologous series is formed by adding a carbon and two hydrogen atoms or a CH2 (methylene group) to the previous molecule. The incremental change in relative molecular mass is therefore fourteen.
The table below shows the first eight straight chained alkanes in the alkane homologous series.
| Name | Number of Carbon atoms | Molecular Formula CnH2n+2 |
Structural Formula | Boiling Point °C | Melting Point °C |
|---|---|---|---|---|---|
| Methane | 1 | C1H2(1)+2 = CH4 | 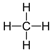 |
−162 | −183 |
| Ethane | 2 | C2H2(2)+2 = C2H6 | 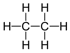 |
−89 | −172 |
| Propane | 3 | C3H2(3)+2 = C3H8 | 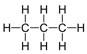 |
−42 | −188 |
| Butane | 4 | C4H2(4)+2 = C4H10 | 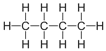 |
−0.5 | −135 |
| Pentane | 5 | C5H2(5)+2 = C5H12 | 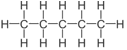 |
36 | −130 |
| Hexane | 6 | C6H2(6)+2 = C6H1 | 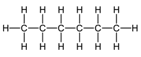 |
69 | −95 |
| Heptane | 7 | C7H2(7)+2 = C7H16 | 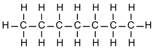 |
98 | −91 |
| Octane | 8 | C8H2(8)+2 = C8H18 | 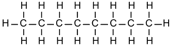 |
126 | −57 |
