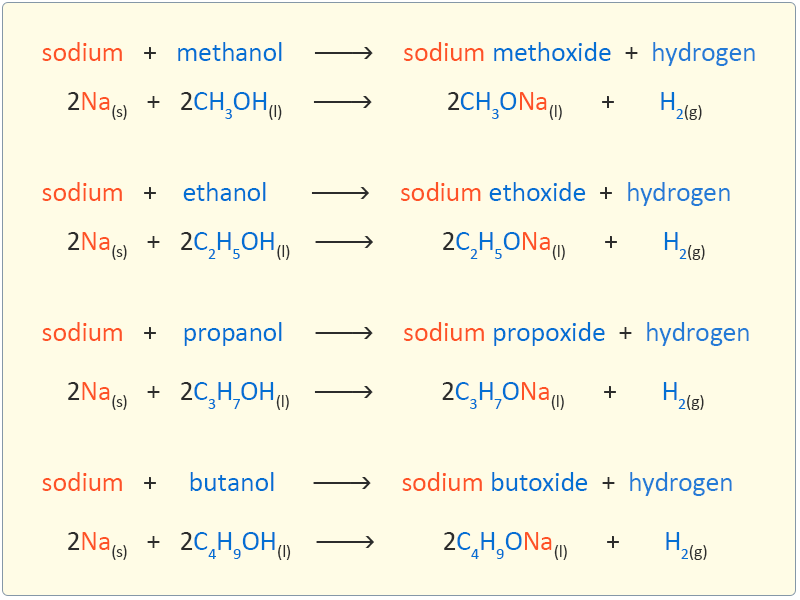
Reaction of Alcohols with Sodium
Alcohols react with sodium to form a salt (sodium alkoxide) and hydrogen gas. The reaction is similar but much slower than the reaction of water and sodium. This is because of the similarities in the structure of the water molecule and the alkyl (O—H) group in alcohols. Due to the low density of the alcohols the sodium sinks. The reaction proceeds steadily with the evolution of hydrogen gas and leaves a colourless solution of the salt. The salt can be recovered as a white solid by careful evaporation of the solution.
The equations for the reaction of the first four alcohols with sodium are given below: