Oxidation of Butanol
Alcohols can be oxidised by a variety of oxidising agents. Sodium or potassium dichromate acidified with dilute sulphuric acid can bring about oxidation in straight chained alcohols. Straight chained alcohols with one alkyl group or primary alcohols as they are referred to can be oxidised to form aldehydes. The resulting aldehyde can then undergo further oxidation to a carboxylic acid.
Remember, oxidation is a process involving the gain of oxygen, loss of hydrogen or loss of electrons. Oxidation of alcohols is oxidation in terms of hydrogen transfer. The alcohol is oxidised by loss of hydrogen. Oxidation and reduction in terms of hydrogen transfer is common in hydrocarbon chemistry.
Butanol is oxidised by sodium dichromate (Na2Cr2O7) acidified in dilute sulphuric acid to form the aldehyde butanal.
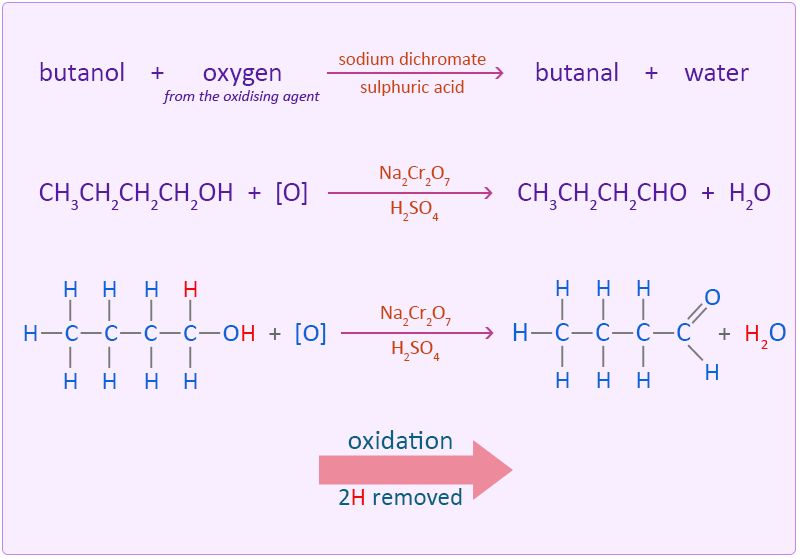
The oxidation of the alcohol to an aldehyde is indicated by the colour change of the dichromate solution as it is reduced from the orange colour of Cr2O72− to the green of chromium(III) ions (Cr3+). In this reaction the butanol is oxidised to butanal by removing two hydrogen atoms.
The aldehyde needs to be removed from the reaction mixture as soon as possible otherwise the resulting aldehyde can undergo further oxidation to a carboxylic acid. This is done by using an excess of alcohol and distilling off the aldehyde as soon as it forms. If however, an excess of the oxidising agent is added and the reaction is allowed to continue with no removal of the aldehyde further oxidation to a carboxylic acid takes place.
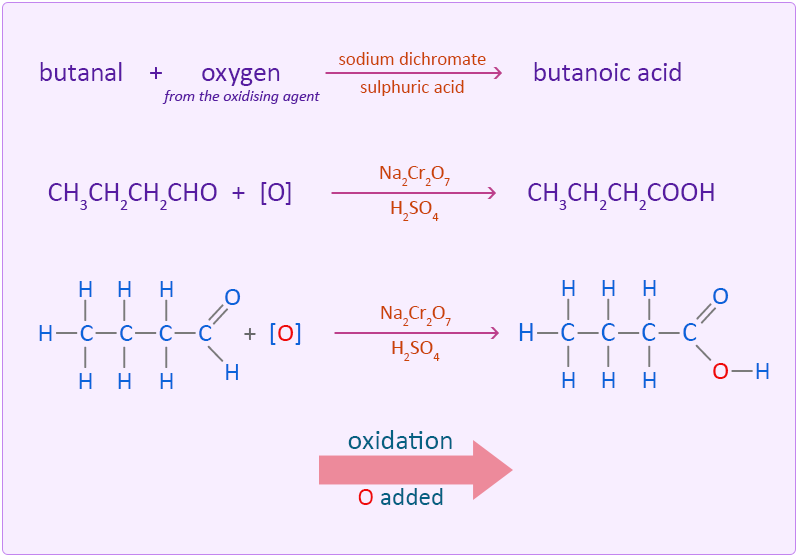
Butanal is oxidised to butanoic acid by adding an oxygen atom.
