Oxidation of Ethanol
Alcohols can be oxidised by a variety of oxidising agents. Sodium or potassium dichromate acidified with dilute sulphuric acid can bring about oxidation in straight chained alcohols. Straight chained alcohols with one alkyl group or primary alcohols as they are referred to can be oxidised to form aldehydes. The resulting aldehyde can then undergo further oxidation to a carboxylic acid.
Remember, oxidation is a process involving the gain of oxygen, loss of hydrogen or loss of electrons. Oxidation of alcohols is oxidation in terms of hydrogen transfer. The alcohol is oxidised by loss of hydrogen. Oxidation and reduction in terms of hydrogen transfer is common in hydrocarbon chemistry.
Ethanol is oxidised by sodium dichromate (Na2Cr2O7) acidified in dilute sulphuric acid to form the aldehyde ethanal.
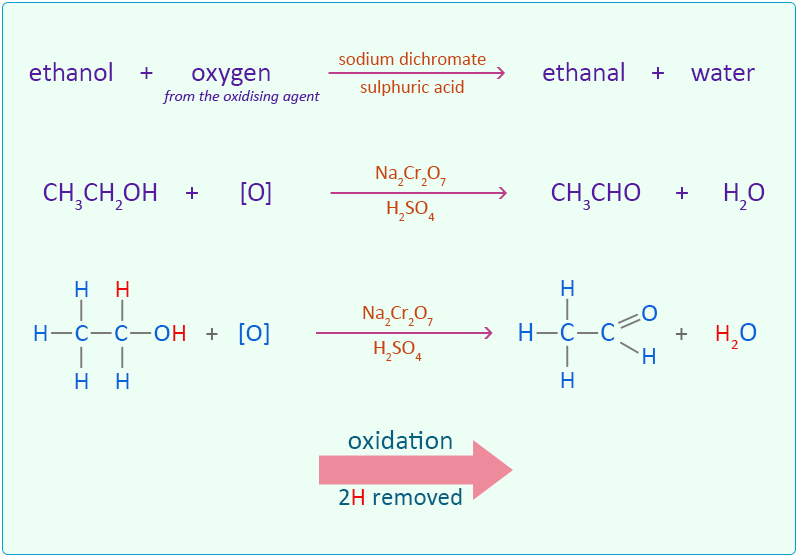
The oxidation of the alcohol to an aldehyde is indicated by the colour change of the dichromate solution as it is reduced from the orange colour of Cr2O72− to the green of chromium(III) ions (Cr3+). In this reaction the ethanol is oxidised to ethanal by removing two hydrogen atoms.
The aldehyde needs to be removed from the reaction mixture as soon as possible otherwise the resulting aldehyde can undergo further oxidation to a carboxylic acid. This is done by using an excess of alcohol and distilling off the aldehyde as soon as it forms. If however, an excess of the oxidising agent is added and the reaction is allowed to continue with no removal of the aldehyde further oxidation to a carboxylic acid takes place.
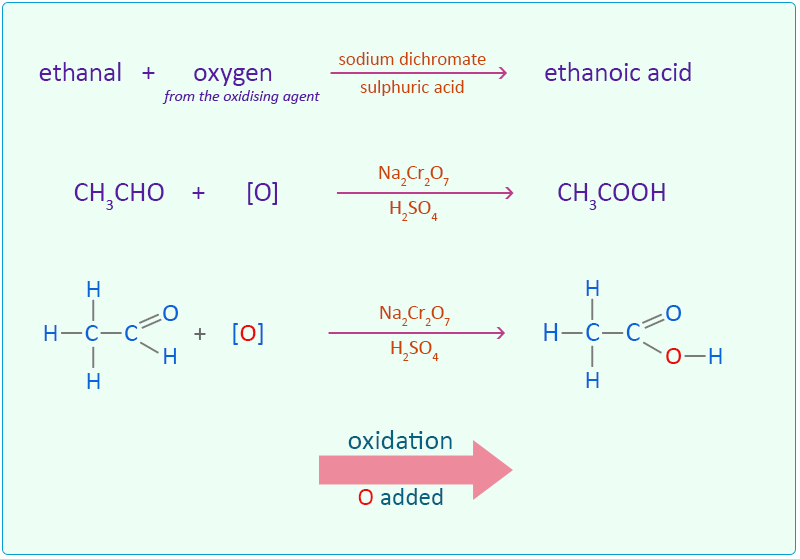
Ethanal is oxidised to ethanoic acid by adding an oxygen atom.
The oxidation of ethanol by an acidified dichromate solution was used in earlier forms of breath alcohol testing devices (breathalysers) to determine the blood alcohol concentration in drink driving suspects. The presence of ethanol is detected by a change in colour of the dichromate solution from orange to green. The degree of the colour change is related to the level of alcohol expelled from the suspect’s breath.
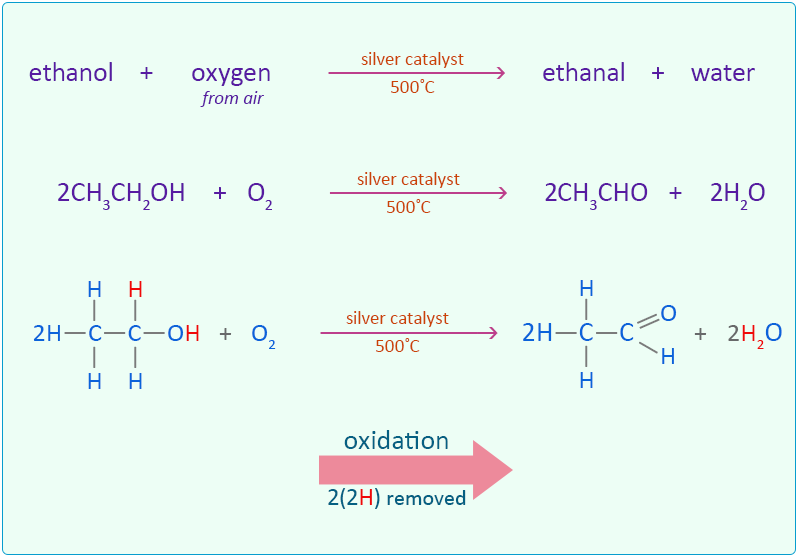
Ethanol can also be oxidised by passing a mixture of ethanol vapour and air over a silver catalyst at 500°C.
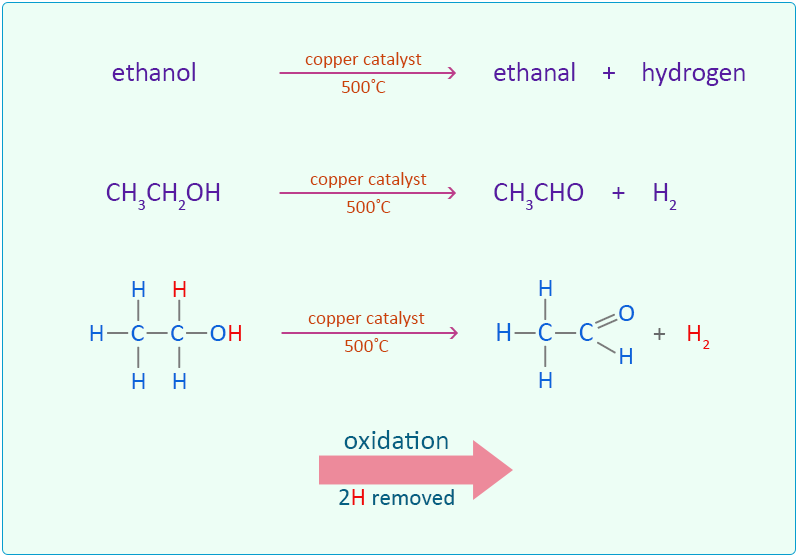
Another industrial process for the oxidation of ethanol to ethanal is by passing ethanol vapour alone over a heated copper catalyst.
Ethanol also undergoes bacterial oxidation to ethanoic acid. This form of oxidation is a problem for wine producers. Air contains a large proportion of bacteria called Acetobacter. Acetobacter bacteria use atmospheric oxygen from air to oxidise ethanol in wine, producing a weak solution of ethanoic acid (vinegar). So once a bottle of wine has been opened it can quickly turn to vinegar due to the large number of bacteria in the air. Wines with a high concentration of alcohol or fortified wines such as sherry and port are very resistant to bacterial oxidation. This is because the ethanol concentration is too high for the bacteria to tolerate.
Vessels used for fermenting beer and wine at home are fitted with airlocks. These allow carbon dioxide gas released from the fermenting process to escape but prevent air from entering the vessel.
